An Expert's Answers On Remdesivir, The Drug Fast-Tracked By The FDA To Potentially Treat COVID-19
On May 1, 2020 the Food and Drug Administration (FDA) issued an emergency use authorization for remdesivir for the treatment of COVID-19 in adults and children hospitalized with severe cases. I met the news with a kind of euphoria. It felt like a huge leap in the right direction.
Since then, I’ve come to realize my euphoria might have been a little overboard—call it, a case of blind hope drenched in a large dose of cabin fever. But, the antiviral drug produced by Gilead Sciences, Inc. (yes, if your brain shot straight to Handmaid’s Tale you’re not alone) is promising, and it is a step in the right direction. And when it comes to COVID-19, we need all the steps in the right direction we can get.
Scary Mommy spoke with Dr. Katie Passaretti, who serves as the Medical Director of Infection Prevention for CMC and is an attending faculty physician in the Department of Internal Medicine, to get a better understanding of remdesivir, as well as to understand the latest information on the various strains of the novel coronavirus.
What Is Remdesivir?
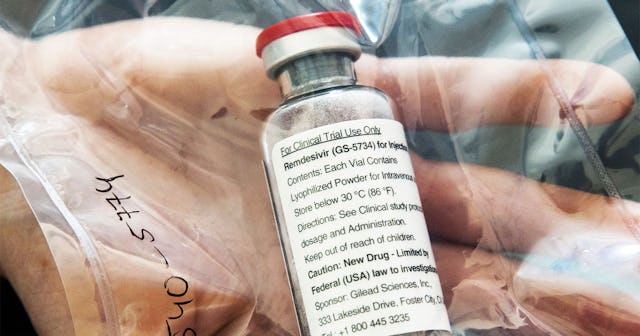
Remdesivir is an antiviral medication that is administered via infusion. Its function is to keep the virus from replicating, and hopefully help patients get better faster.
Who Is Remdesivir For?
One vial of the drug Remdesivir lies during a press conference about the start of a study with the Ebola drug Remdesivir in particularly severely ill patients at the University Hospital Eppendorf (UKE) in Hamburg, northern Germany on April 8, 2020, amidst the new coronavirus COVID-19 pandemic. ULRICH PERREY/Getty
As of this writing, remdesivir has emergency use authorization only for hospitalized, severe cases of COVID-19. In theory, that seems clear cut. In practice, doctors are required to make decisions on a case-by-case basis to determine which patients will receive remdesivir due to the availability of the drug. (Dr. Passaretti noted that most doctors are open to treating patients with the drug, if they had it, and Gilead is currently working with other manufacturers to figure out how to increase production to meet the anticipated demand.)
Dr. Passaretti’s hospital system received a small stockpile of the drug under the emergency use authorization and is actively using the medication on patients. A team of experts examines factors such as oxygen needs and levels of viral load, and then determines on a case-by-case basis which patients they believe are most likely to respond well to the drug — and it’s not always clear who that might be. Hospitals are still learning who benefits the most from the antiviral drug, and Dr. Passaretti said she’s been “surprised by who it helps and who it doesn’t.”
The one truth that seems to be emerging so far is that it’s important to get the medication earlier in the infection cycle, rather than later.
Researchers are also currently studying the benefits of remdesivir in moderate, hospitalized cases.
Is Remdesivir a Cure?
Unfortunately, no. Remdesivir is a promising treatment, but not a “knockout” in the words of Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases.
Studies have shown that remdesivir shortens the time for recovery, from 15 days, in patients who received a placebo, to 11 days in patients who received the emergency use treatment. However, the mortality rate between the placebo group and the remdesivir group was not statistically significant.
So, yes, promising, because remdesivir is proof that the virus can be blocked, but not yet the miracle cure I had hoped when news first broke.
What Are the Side Effects of Remdesivir?
Gilead Sciences headquarters sign is seen in Foster City, California on April 30, 2020. – Gilead Science’s remdesivir, one of the most highly anticipated drugs being tested against the new coronavirus, showed positive results in a large-scale US government trial, the company said on April 29, 2020. JOSH EDELSOnN/Getty
Remdesivir may cause low blood pressure, nausea, vomiting, sweating, and shivering during the infusion. It may also cause an increase in levels of liver enzymes, and Dr. Passaretti urged caution in treating a patient with remdesivir who already had liver dysfunction.
Dr. Passaretti noted that she and her team were learning as they go (as seems to be the case in every aspect of this virus) when it comes to side effects. The more they use the medication and learn about its side effects, the more informed each subsequent decision will be.
What’s Next for Remdesivir?
An exciting partnership. (Or that’s my blind hope drenched in a large dose of cabin fever rearing its ugly head again.)
Researchers are currently conducting a trial looking at remdesivir in combination with immune calming agents. The hope is that the combination would be more effective than just the antiviral alone, because in many severe cases, the individual’s immune response can be as damaging a the virus itself.
Dr. Aneesh Mehta, the lead investigator of the National Institute of Health trial at Emory University, explained the approach to CBS News. She said, “What the remdesivir here does is stops the spark and the immune modulator will hopefully be putting dirt on the fire to put it out. A one-two punch.”
Is COVID-19 Mutating?
Yes, but that’s not a reason to panic. Respiratory viruses mutate, and in this respect COVID-19 is not an outlier. According to Dr. Passaretti, the virus does seem to be mutating slower than some other viruses. “We’re still learning how this virus acts, but the [variety of symptoms we’re seeing are] less likely a strain issue and just that this virus has characteristics we’re still learning about.
With respect to the news that one strain may be more contagious, Dr. Passaretti notes that the rate of transmission has been largely static, and it’s unclear how that plays out.
Ultimately, the big question we all want answered is when can we get back to normal? The road to a vaccine will likely be a long one, despite a handful of hopeful trials coming down the pipeline, and we all want to know if there’s a way out sooner—is remdesivir that way “out” we’re all searching for?
As it stands now, remdesivir is not that way out—meaning, it’s not a reason to throw social distancing measures out the proverbial window. Even though remdesivir has effectively given us hope that COVID-19 can be blocked, and even though it seems to shorten recovery time in the most severe cases, it cannot stop the influx of cases hospitals would undoubtedly see if all social distancing measures were dropped.
But still, it’s a reason to hope. And to be proud of how far we’ve come in this fight against a virus that doesn’t always play fair.
This article was originally published on