Moderna Begins Testing COVID Vaccine In Babies As Young As 6 Months
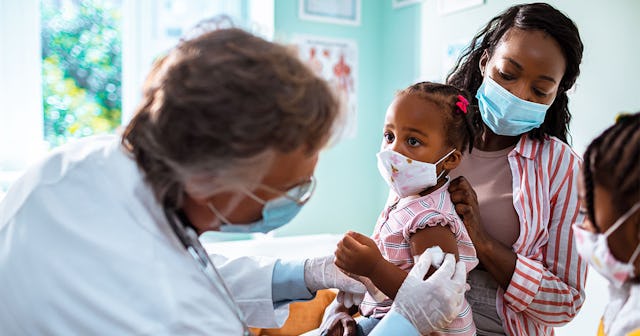
The Moderna study is expected to enroll 6,750 healthy children in the United States and Canada
Pharmaceutical company Moderna announced it has officially begun a study to test its COVID vaccine in children under 12, which includes babies as young as six months old. Clinical trial participants will consist of healthy children throughout the U.S. and Canada.
“There’s a huge demand to find out about vaccinating kids and what it does,” said Dr. David Wohl, the medical director of the vaccine clinic at the University of North Carolina, tells the New York Times.
The study expects to enroll 6,750 children to receive two shots, 28 days apart. Children in this study who are between the ages of two and 12 may receive two doses of 50 or 100 micrograms each. Children under two may receive two shots of 25, 50, or 100 micrograms.
This study is welcome news for parents across the country who want their children to receive protection through vaccination as millions of adults already are. Dr. Fauci and other epidemiologists have made public statements about how crucial vaccinating children is to achieving herd immunity. The American Academy of Pediatrics has called for expansion of vaccine trials to include children.
In a separate study, Moderna is testing the vaccine in 3,000 children between the ages of 12 and 17. Results are expected in regard to that age group as soon as this summer. If the results show the vaccine is safe and effective for children, it will then have to be authorized for use in children before it can be distributed.
In December 2020, the U.S. Food and Drug Administration approved the Pfizer-BioNTech vaccine for 16 and 17-year-olds as well as adults. Currently, Moderna is only approved for those 18 and up.
In each study for the Moderna vaccine, the first children to be inoculated will receive the lowest doses of the vaccine and will be monitored for reactions before other participants are given higher doses. Researchers will then perform an analysis to determine which dose is safest for each age group. In part two of the study, the kids who participate will receive doses based on that analysis (or placebo shots).
After receiving their vaccines, the children in the study will be followed for a year so experts can look for side effects and also measure antibody levels that will help determine the level of protection from the vaccine.
Estimations for when children will be able to be vaccinated vary; the estimated timeline for older kids could be as early as this fall. Younger children likely won’t be able to be vaccinated until late 2021 or early 2022.
Moderna’s child study, which is coordinated in conjunction with the National Institute of Allergy and Infectious Diseases, is officially underway one year after the first adult participant got a shot of the Moderna vaccine during initial trials last March.