What Parents Should Know About The New COVID Variant Boosters Just Approved For Kids
Kids can get the updated booster two months after their initial shots or their last booster.
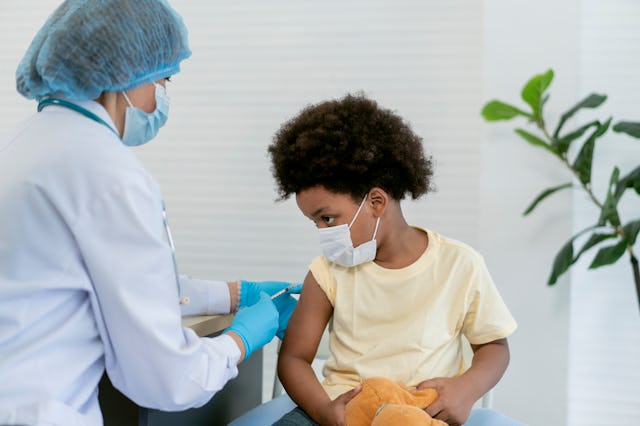
Both the Food and Drug Administration (FDA) and the Centers For Disease Control and Prevention (CDC) have approved and authorized the updated COVID-19 booster shots for kids over the age of 5 — and shots are immediately available.
Within hours of each other, the two federal organizations gave their okay, allowing most kids access to the boosters, which provide greater protection against the Omicron variant, ahead of winter and the travel-heavy holidays.
Specifically, the FDA authorized Pfizer's updated vaccine for kids 5 to 11 while Moderna’s variant shot is cleared for kids 6 through 17.
The vaccine protects against both the BA.4 and BA.5 omicron subvariants — currently, about 79% of cases in the U.S. are the BA.5 subvariant.
“As we head into the holidays, where we expect families will gather together, people of all ages may be more prone to exposure to the COVID-19 virus,” Dr. Hilary Marston, Chief Medical Officer of the FDA, tells Scary Mommy. “We know that staying up to date on vaccines is the best way to protect our families against COVID-19 and its most devastating consequences. But we also know the virus has evolved. These updated vaccines target the most recent Omicron variants, as well as protecting against the original strain of the virus.”
Dr. Marston stresses that while COVID-19 has historically been mild in children, the booster can still greatly reduce hospital visits, long-term symptoms, and community spread.
“While COVID-19 tends to be less severe in children than adults, many children have gotten sick with the disease and have been hospitalized,” she explains. “Children may also experience long-term effects of COVID-19. The new updated vaccines are critical to helping protect our kids from the most serious outcomes of COVID-19 caused by currently circulating variants — especially as we head into the fall and winter.”
She also points out that children’s immunization rates have greatly lagged behind adult vaccination rates — and that now is an optimal time to start the vaccination processes with your kids if you haven’t already. Currently, only about one in three kids ages 5 to 11 have been vaccinated.
“We are also aware that many children have not yet had their primary COVID-19 vaccination, and we encourage parents and caretakers to consider primary vaccination for children and follow-up with an updated vaccines dose for their children when they are eligible,” she says.
Marston also points out that the updated booster has been studied and tested and is safe for kids.
For each of the updated vaccines authorized, the FDA took into consideration several different types of data: first, clinical studies of bivalent boosters in adults with a related Omicron strain showed the safety and robust immune response that these updated boosters would provide. Second, clinical studies of monovalent (or single strain) boosters, including for pediatric age groups, which again reassured the FDA about safety and ability to provide a strong immune response. And of course, FDA took into account the real world data on the original COVID-19 vaccines, which have been administered to hundreds of millions of people around the world, including young children.
Who is eligible for the shot among kids over 5 years of age? Anyone who received their second booster shot at least two months ago. Those who have tested positive for COVID shouldn’t get their booster for three months.